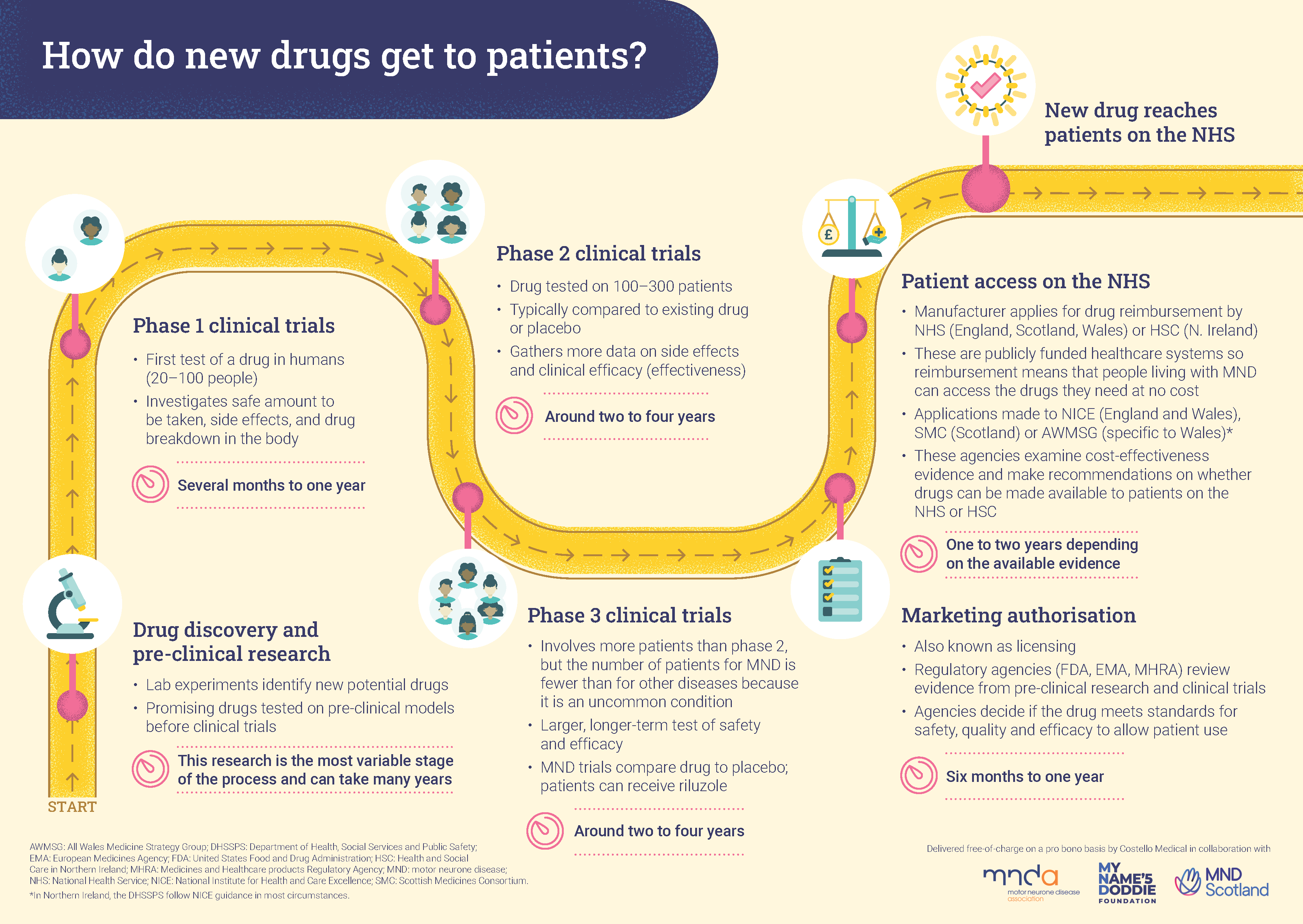
The clinical trials process: how MND drugs are discovered, developed and approved in the UK
A drug or treatment’s journey from initial development in the lab to being prescribed by doctors can take many years and involves several stages which help researchers understand whether the drug is safe, effective and causes any side effects.
The MND Association has worked collaboratively with MND Scotland and My Name’5 Doddie Foundation, with support from Costello Medical, to create the following offering clear, concise information about the clinical trials process in the UK.
What is the process for repurposed drugs?
Repurposing drugs is a way to find new uses for medicines already available on prescription. Around one-third of newly approved treatments are already being used to treat a different medical condition.
Drug repurposing is based on the principle that one drug can affect multiple biological processes so may be useful for treating multiple conditions. For instance aspirin was originally used as a painkiller but has been repurposed to reduce the risk of heart attacks and strokes, because it also thins blood.
As these drugs have been previously tested and used in people, we already know they’re safe so the drug can skip the initial stages of testing and move straight to later phase clinical trials. That can save years of development time, save money, and decrease the risk of initial failure.
As with any drug trial. there must be strong evidence that a repurposed drug has a chance of working on the particular disease being targeted. And the drug has to be tested just as rigorously as a new drug.
With medicine repurposing offering a quicker path to testing potential treatments for MND, these kinds of trials are an important part of the our research funding portfolio.